![]()
Soricimed is using TRPV6-targeting peptide ligands to develop next generation peptide-drug conjugates for cancer treatment. Many potent cancer-killing drugs used in cancer treatment do not discriminate tumour tissue from healthy tissue resulting in the potential for off target toxicities. To overcome these limitations, we link these highly potent cytotoxic payloads to peptides that specifically target TRPV6 over-expressed in cancerous tissues to deliver the payload quickly and directly to the tumor. As a result, the cytotoxic cancer drug rapidly accumulates in the tumor and spares healthy tissue from exposure to these toxic agents. We are currently investigating several TRPV6-targeting peptide-drug conjugates that have shown very encouraging tumor responses in preclinical cancer models.
A tumor-targeting peptide binds to TRPV6 receptors, which are overexpressed on cancer cells, concentrating the peptide-drug conjugate (PDC) at the cell surface. This complex is then internalized through endocytosis, forming vesicles that mature into endosomes. The cytotoxic drug is released either within lysosomes, late endosomes, the cytosol (after escape), or at the membrane if cleavable linkers are used. Once released, the drug attacks vital cellular components like DNA, microtubules, or topoisomerases, ultimately causing cancer cell death as shown in the graphic below.
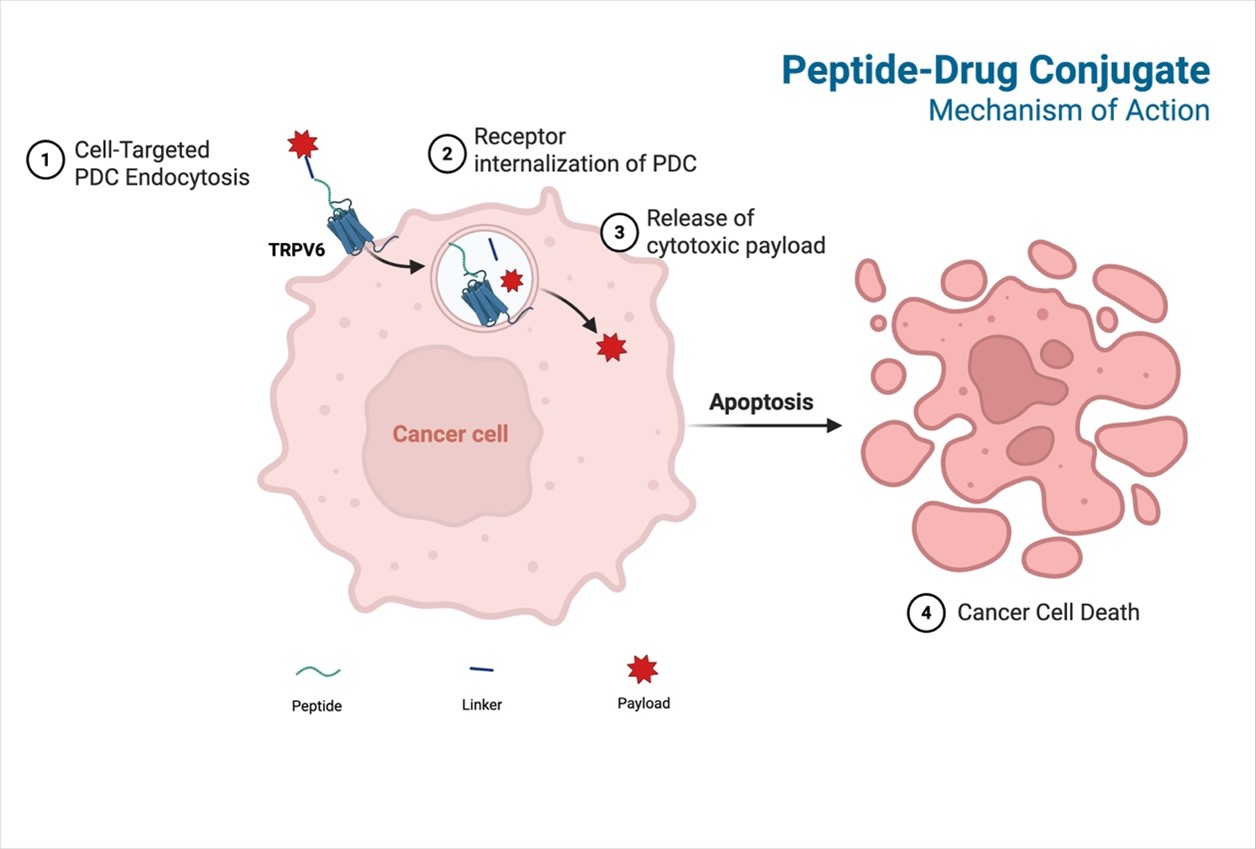
Our peptide-drug conjugate (PDC) approach has several advantages over other drug conjugate programs like antibody-drug conjugates (ADCs).2
References:
1 Acta Pharm Sin B. 13(2):498-516 (2023)
2 J Nanobiotechnology 22;23(1):305 (2025)
Address
18 Botsford St., Suite 201
Moncton, N.B. E1C 4W7
Canada
T: 506.856.0400
F: 506.856.0414
E: info@soricimed.com
© Copyright 2025 Soricimed Biopharma