![]()
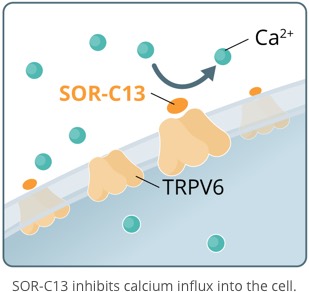
SOR-C13, is a novel, short, synthetic peptide developed from the C-terminal region of Soricidin, our proprietary 54 amino acid peptide. SOR-C13 binds with affinity and selectivity to - and disrupts the function of - TRPV6, a calcium channel over-expressed in solid tumor cancers. TRPV6 plays a central role in a biochemical cascade that results in the upregulation of an array of pro-cancerous genes. TRPV6 is considered to be an important target for novel anticancer therapy. SOR-C13 has been granted orphan drug status by the U.S. Food and Drug Administration for the treatment of pancreatic and ovarian cancers. It is the first highly specific TRPV6 inhibitor to be identified and to be taken into clinical development.
Our ‘first-in-human’ Phase 1 clinical trial, was a dose escalation safety study evaluating SOR-C13 in adults with advanced solid tumors of epithelial origin non-responsive to all standard-of-care treatments (NCT01578564). We enrolled 23 patients - 22 patients with stage 4 cancer and one patient with stage 3 cancer. In total, there were 14 different solid tumor cancers represented. The study took place at the University of Texas MD Anderson Cancer Centre in Houston, Texas, the Juravinski Cancer Center in Hamilton, Ontario, and the London Health Sciences Centre in London, Ontario.



Patients were treated on a set schedule of IV dosing for two 21-day cycles. At the end of the second cycle if their physician felt the patient was receiving a treatment benefit without unacceptable side effects, the duration of treatment could be expanded using the same 21-day treatment cycles.
![]()
The results showed SOR-C13 was safe and generally well tolerated in the FIH trial patients, without evidence of the hematological, cardiac, neurological or other significant toxicities often observed with cytotoxic chemotherapy. Because of the expanded treatment duration, a total of 486 IV infusions were given in the Phase 1 trial. With this, there were no drug related serious adverse events reported.
The FDA has awarded orphan drug status to SOR-C13 for the treatment of ovarian cancer and for the treatment of pancreatic cancer.
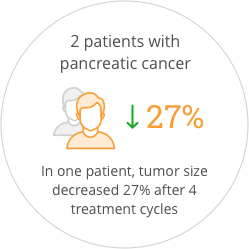
The study also provided preliminary indications of anticancer activity for SOR-C13. After the first two cycles of treatment, 55% of patients had stable disease. One patient that came into the study with progressive disease was stable for more than one year. Promising anti-tumor activity was seen in both patients with pancreatic ductal adenocarcinoma, who had each failed three prior regimens of anticancer therapy. One of these patients showed a 27% reduction in tumor size after four treatment cycles which correlated with a validated blood biomarker for pancreatic cancer. The FDA has awarded orphan drug status to SOR-C13 for the treatment of ovarian cancer and for the treatment of pancreatic cancer.
For a comprehensive description of our Phase 1 clinical trial please see the attached paper published in the journal Investigational New Drugs.
The absence of toxicity commonly observed with cytotoxic agents provides a rationale for the investigation of SOR-C13 with standard of care antineoplastic agents (i.e., combination with other cancer drugs) to improve anticancer efficacy with reduced risk of cumulative or overlapping toxicities.
Address
18 Botsford St., Suite 201
Moncton, N.B. E1C 4W7
Canada
T: 506.856.0400
F: 506.856.0414
E: info@soricimed.com
© Copyright 2025 Soricimed Biopharma