![]()
ONCOLOGY





Soricimed’s oncology vertical focuses on targeted drug candidates for the treatment of solid-tumor cancers. Soricimed’s lead drug candidate, SOR-C13, has been shown to be safe and well tolerated, with indications of efficacy in a Phase 1 human clinical trial. A follow on Phase 1b Investigator Initiatied Trial (“IIT”) in late-stage cancer is currently underway. SOR-C13 has been granted orphan drug status for the treatment of pancreatic and ovarian cancers by the U.S. Food and Drug Administration. In addition, a portfolio of targeted Peptide-drug Conjugates (“PDCs”) is in late pre-clinical development at this time.
Initiated September 2019


Completed 2017



![]()
![]()
The Transient Receptor Potential (TRP) channels are a superfamily of cation-permeable ion channels. TRPV6 (Subfamily Vanilloid, Member 6) is specific for calcium channels and is implicated in the development and progression of numerous forms of cancer.
This "onco-channel" has emerged as a new target for treating and diagnosing various carcinomas.
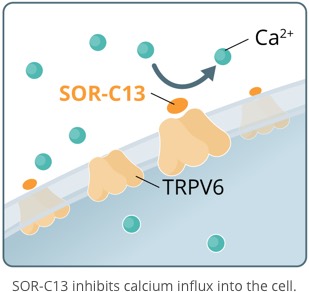
TRPV6 plays a key role in cancer pathogenesis. SOR-C13 disrupts the function of TRPV6.
Peptide Drug-Conjugate (PDC) Programs
Soricimed's PDCs are sequentially assembled constructs that combine:
(e.g. Soricimed Peptide
Which introduces specificity, enabling cytotoxic drug delivery to TRPV6 over expressed by tumors, while minimizing unwanted toxicities from non-specific distribution and death of healthy cells

Linking the targeting ligand (Soricimed Peptide) to a cytotoxic drug

For tumor destruction
An Exciting New Application
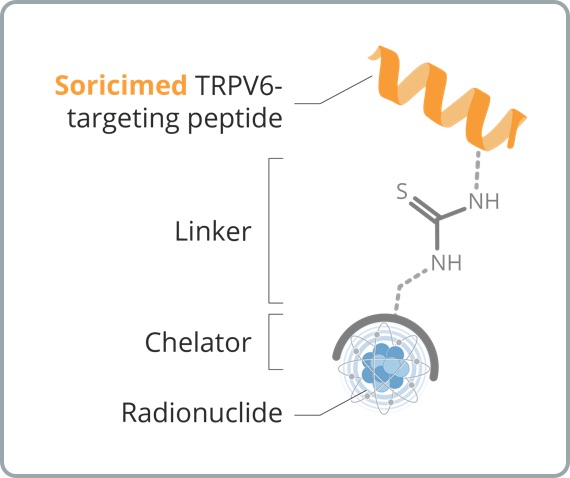
Peptide Receptor Radionuclide Therapy (“PRRT”), involves targeted delivery of isotopes that emit highly energetic particles to cancer cells leading to their destruction, while minimizing collateral damage to healthy surrounding cells.
PRRT is attracting interest as therapeutic oncology class due to increasing availability of wide range of radionuclides, and recent advances in targeting therapies such as mAbs -> “Weaponized Targeted Therapy”.
 SOR-C13, is a novel, short, synthetic peptide developed from the C-terminal region of soricidin, a proprietary 54 amino acid peptide, discovered by Soricimed Biopharma found in the saliva of the Northern Short-tailed Shrew. SOR-C13 binds with high affinity and selectivity - and disrupts the function of - TRPV6, a calcium channel over-expressed in solid tumor cancers. TRPV6 plays a central role in a biochemical cascade that results in the upregulation of an array of pro-cancerous genes. TRPV6 is considered to be an important target for novel anticancer therapy. SOR-C13 is the first highly specific TRPV6 inhibitor to be identified and to be taken into clinical development.
SOR-C13, is a novel, short, synthetic peptide developed from the C-terminal region of soricidin, a proprietary 54 amino acid peptide, discovered by Soricimed Biopharma found in the saliva of the Northern Short-tailed Shrew. SOR-C13 binds with high affinity and selectivity - and disrupts the function of - TRPV6, a calcium channel over-expressed in solid tumor cancers. TRPV6 plays a central role in a biochemical cascade that results in the upregulation of an array of pro-cancerous genes. TRPV6 is considered to be an important target for novel anticancer therapy. SOR-C13 is the first highly specific TRPV6 inhibitor to be identified and to be taken into clinical development.
SOR-C13 was effective in inhibition of tumors in animal xenograft models of human ovarian and breast cancer. Studies with fluorescently tagged SOR-C13 injected intravenously (IV) showed rapid uptake by ovarian and prostate xenograft tumors with signals becoming well visible in about 20–30 min, maximizing at about 1 h and maintaining for at least 72 hours. This indicates that SOR-C13 rapidly accumulates within tumors and resides there for several days where its TRPV6 inhibition is exerted.
In rat and dog GLP toxicology studies (required by regulatory bodies to support human clinical trials), where SOR-C13 was injected as a daily IV bolus for 28 days, there was minimal toxicity and no immunogenicity. Importantly there were no toxic effects on the heart in either species. The lack of cardiotoxicity was further substantiated by studies of other channels where there was no modulation of channel activity by SOR-C13. In addition, SOR-C13 could be administered at high doses before any signs of anatomical pathology. The pre-clinical data was submitted to the FDA as an Investigational New Drug Application (IND) and to Health Canada as a Clinical Trial Application (CTA) to support moving SOR-C13 into human clinical trials in cancer patients.
This was a ‘first-in-human’ Phase 1 clinical dose escalation safety study evaluating SOR-C13 in adults with advanced solid tumors of epithelial origin non-responsive to all standard-of-care treatments (NCT01578564). We enrolled 23 patients - 22 stage 4 cancer and one patient with stage 3 cancer. In total, there were 14 different solid tumor cancers represented. The study took place at the University of Texas MD Anderson Cancer Center in Houston, Texas, the Juravinski Cancer Center in Hamilton, Ontario, and the London Health Sciences Centre in London, Ontario.



The results showed SOR-C13 was safe and generally well tolerated in patients with advanced solid tumors of epithelial origin, without evidence of the hematological, cardiac, neurological or other significant toxicities often observed with cytotoxic chemotherapy. Because of the expanded treatment duration, a total of 486 IV infusions were given in the Phase 1. With this, there were no serious drug-related adverse events reported.
![]()
The results showed SOR-C13 was safe and generally well tolerated in patients with advanced solid tumors of epithelial origin, without evidence of the hematological, cardiac, neurological or other significant toxicities often observed with cytotoxic chemotherapy. Because of the expanded treatment duration, a total of 486 IV infusions were given in the Phase 1. With this, there were no serious drug-related adverse events reported.
The FDA has awarded orphan drug status to SOR-C13 for the treatment of ovarian cancer and for the treatment of pancreatic cancer.
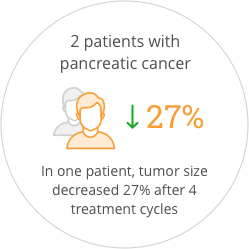
The study also provided preliminary indications of anticancer activity for SOR-C13. After the first two cycles of treatment, 55% of patients had stable disease. One patient that came into the study with progressive disease was stable for more than one year. Promising anti-tumor activity was seen in both patients with pancreatic ductal adenocarcinoma, who had each failed three prior regimens of anticancer therapy. One of these patients showed a 27% reduction in tumor size after 4 treatment cycles which correlated with a validated blood biomarker for pancreatic cancer. The FDA has awarded orphan drug status to SOR-C13 for the treatment of ovarian cancer and for the treatment of pancreatic cancer.
For a comprehensive description of our Phase 1 clinical trial please see the attached paper published in the journal Investigational New Drugs.
SOR-C13 provides a unique mechanism for anticancer activity, through inhibition of the TRPV6 calcium channel activity. Based on good drug tolerability and manageable safety profile, and coupled with promising anti-tumor activity, further studies to investigate SOR-C13 as an anti-cancer agent are planned.
The absence of toxicity commonly observed with cytotoxic agents provides a rationale for investigation of SOR-C13 with background antineoplastic agents (i.e., combination with other cancer drugs) to increase anticancer efficacy with reduced risk of cumulative or overlapping toxicities.
Address
18 Botsford St., Suite 201
Moncton, N.B. E1C 4W7
Canada
T: 506.856.0400
F: 506.856.0414
E: info@soricimed.com
© Copyright 2025 Soricimed Biopharma